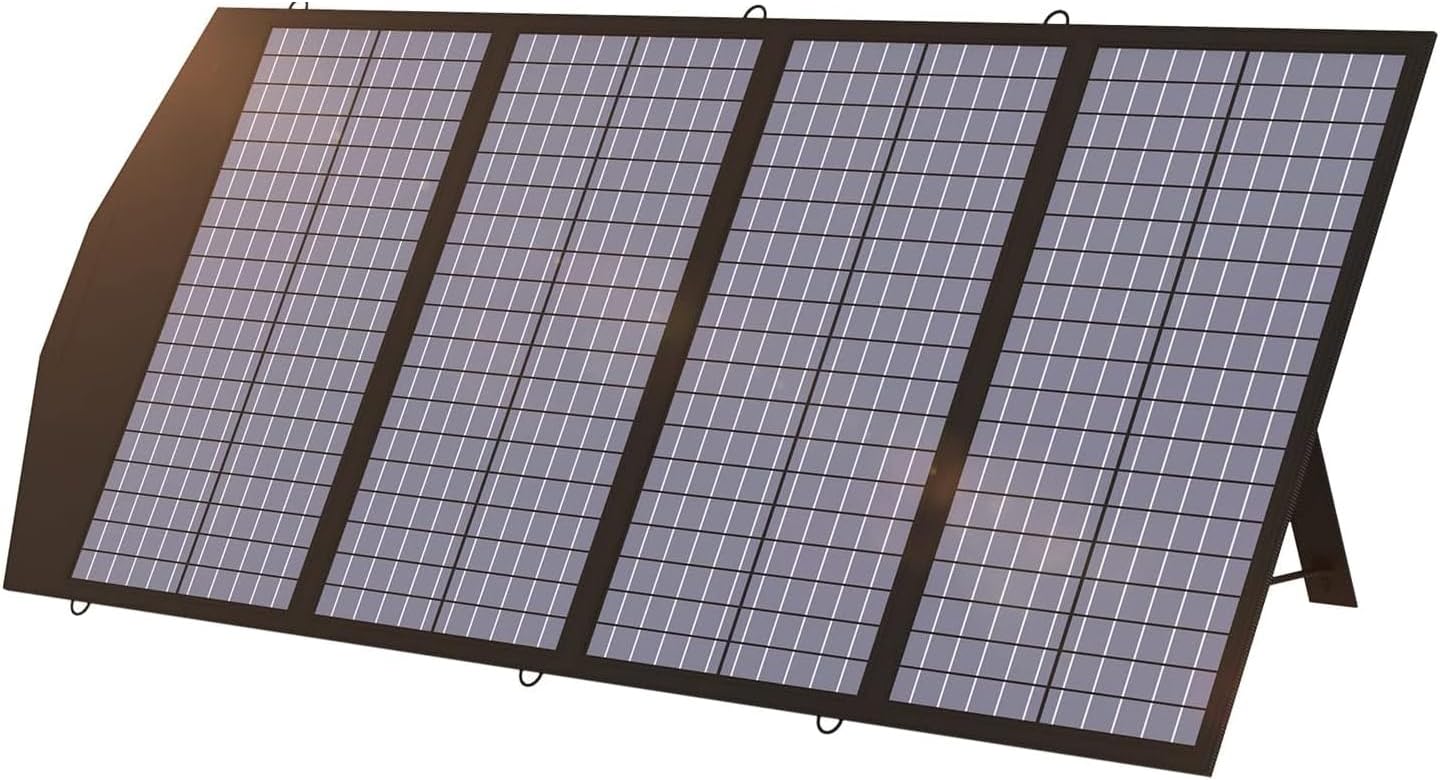
SUNER POWER Waterproof 12W 12V Solar Battery Charger & Maintainer Pro, Built-in UltraSmart MPPT Charge Controller, 12 Volt Solar Panel Trickle Charging Kits for Car Automotive Boat Marine RV Trailer

$53.95
@ Amazon.com (Save 10%)
Product Description【Ultra-Smart MPPT Technology】This 12v solar panel battery charger is crafted with SUNER POWER's unique Ultra-Smart MPPT( Max Power Point Tracking) technology, the innovative MPPT allows to deliver high tracking efficiency of up to 99% and peak conversion efficiency of 98%, improving approximately 20%-30% utilization rate than other competitors
【Improved 3-stages Charging】The improved 3-Stages charging algorithm (Bulk, Absorption, Float) allows to charge 12-volt battery more effectively, ensures battery is safely recharged and increases battery life and performance
【Free Maintenance】The solar trickle charger stops charging when battery has full charge, and automatically resumes when battery has discharge, totally free maintenance. A fully-automatic, worry-free and smart 12volt solar charger
【Full Safety Protections】Built-in multiple protections including over-charge, over-discharge, over-voltage, over-current, over-load, short circuit, reverse polarity, and over-temperature, waterproof and spark-proof, the battery is fully protected
【Wide Compatibility】It's not just a solar powered battery charger, it's an advanced solar battery maintainer for 12-volt sealed lead-acid car, automotive, marine, RV, power sport, boat, dump trailer, motorcycle, trolling motor, tractor, ATV and deep-cycle batteries, including flooded, gel, AGM, SLA and maintenance-free, plus lifepo4 lithium batteries
【Superior Quality and Material】Made of premium Grade A+ solar cells with efficiency up to 21%-30%, tempered solar glass and durable corrosion-resistant frame, withstand extreme weather conditions like UV, anti-rusting, erosion, hail, and sandstorm
Product Details| Brand: | SUNER POWER |
|---|
| Model #: | BC-50W-P |
|---|
| Size: | BC-12W Pro |
|---|
| Color: | Navy Blue |
|---|
| Dimensions: | 16.5 X 9.4 X 0.6 Inches (Length X Width X Height) |
|---|
| Price: | $53.95 (Save 10%) |
|---|
SUNER POWER Waterproof 12W 12V Solar Battery Charger & Maintainer Pro, Built-in UltraSmart MPPT Charge Controller, 12 Volt Solar Panel Trickle Charging Kits for Car Automotive Boat Marine RV Trailer
ECO-WORTHY 10W Solar Car Battery Charger Maintainer 12V Waterproof Portable Solar Trickle Charger for Car Truck Boat Lawn Mower RV Trailer Tractor ATV Utility Vehicle Battery
Product Description【Function】Suitable for keeping vehicles/boat battery charged during the weeks unused. No need to disconnect the battery or have someone start the vehicle periodically. The indicator light on the back can observe the charging state. And it’s waterproof, you can safely use it outdoors(After experimental test).
【Protection】The 10w monocrystalline solar panel uses trickle charging to charge the battery without causing overcharging. Built-in blocking diode prevents reverse charging without extra maintenance at night. Works even on cloudy days. As frosted surface, the panel doesn't need film protection anymore.
【Solar Trickle Charger Upgrade】PCB upgrade and addition the function of reverse and overcharge protections.And you can test the open circuit voltage normally.ECO-WORTHY 10W solar battery charge your car battery more safely and efficiently.Be a good helper on your journey!
【Easy to Install】Equipped with SAE cable, just plug the cigarette lighter into the port or connect the battery terminal posts with the alligator clip, and the connection can be completed within 3 minutes! Suitable for car, truck, boat, lawn mower, motorcycles, trailer, dump trailer, tractor, RV, van, camper, ATV, utility vehicle Battery etc.
【Good Service】If you have any questions, please contact us. Your suggestion helps us improve!
Product Details| Brand: | ECO-WORTHY |
|---|
| Model #: | L02EP10BB18V-1 |
|---|
| Size: | 10W |
|---|
| Color: | Black |
|---|
| Dimensions: | 1.40157480172 X 9.59842518706 X 15.29921258282 inches (Length X Width X Height) |
|---|
| Price: | $42.99 |
|---|
ECO-WORTHY 10W Solar Car Battery Charger Maintainer 12V Waterproof Portable Solar Trickle Charger for Car Truck Boat Lawn Mower RV Trailer Tractor ATV Utility Vehicle Battery
POWOXI Upgraded 7.5W-Solar-Battery-Trickle-Charger-Maintainer-12V Portable Waterproof Solar Panel Trickle Charging Kit for Car, Automotive, Motorcycle, Boat, Marine, RV,Trailer, Snowmobile, etc.
Product DescriptionUpgrade Intelligent Charge Controller: Built-in internal protection system and smart fast charging Cooperate with Quality solar silicon material ensures high conversion and stable output the solar panel's energy conversion efficiency to 25-30%
Smart Internal Protection System: From POWOXI technology Solar battery charger will not damage the battery and overcharge. The built-in smart protection system and blocking diode structure ensures no overvoltage and short circuit. Make charging safer
Stronger & More Long-lasting: The 7.5W solar car battery charger adopts high-transparency glass, which is high-strength, impact-resistant, wind-snow-resistant low-iron tempered. This waterproof solar battery charger needs no additional maintenance
Scratch Protection Design: The ABS+PC frame is stronger than flexible solar panels, so there's no need to worry about the solar panels bending. Aluminum alloy may damage the car paint, but with the ABS+PC frame, for durability this is not a concern
Easy to Install and Use: The solar battery maintainer is easy to install, and convenient to use, just insert it in the car power outlet, as known as the cigar lighter socket or connect the solar panel to the battery with the alligator clips directly
Wide Range of Applications: The perfect charger/maintainer for any car, LiFePO₄, Lithium Ion, motorcycle, boats, tractor, ATV, farm device, and 12V batteries, With a POWOXI solar charger, you don't have to worry about battery drain
Product Details| Brand: | POWOXI |
|---|
| Model #: | ABS Solar 7.5W |
|---|
| Warranty: | 1 year |
|---|
| Size: | Solar Powered Battery Chargers 7.5w-B |
|---|
| Color: | Solar Powered Battery Chargers 7.5w-B |
|---|
| Dimensions: | 14.7637795125 X 1.8897637776 X 9.055118101 Inches (Length X Width X Height) |
|---|
| Price: | $39.99 |
|---|
POWOXI Upgraded 7.5W-Solar-Battery-Trickle-Charger-Maintainer-12V Portable Waterproof Solar Panel Trickle Charging Kit for Car, Automotive, Motorcycle, Boat, Marine, RV,Trailer, Snowmobile, etc.
Saillong 10W 12V Solar Car Battery Charger Maintainer, Waterproof Solar Panel Portable Solar Trickle Charger with Cigarette Lighter Plug Alligator Clip for Car Motorcycle Boat Marine RV

$18.99
@ Amazon.com (Save 10%)
Product DescriptionSuitable for all rechargeable 12v batteries: As a solar battery charger and maintainer, it can safely maintain 12v batteries, suitable for keeping vehicle/boat batteries charged when not used for weeks, without having to disconnect the battery or have someone start the vehicle regularly
Easy to use: Heavy duty 2.1mm x 5.5mm DC plug with extension cord, plug and play, no additional maintenance, no electricity bills, as long as facing the sun, the charger will generate current to trickle charge the battery; Comes with a carabiner and suction cup to meet your different installation needs
Intelligent protection: built in charging microprocessor, improved charging algorithm, can provide overvoltage protection, overcharge protection, short circuit protection, etc., can prevent reverse charging, can be safely used for charging and maintenance of 12V batteries, maintain battery life and extend its service life, prevent battery exhaustion
Easy to Install: Equipped with SAE cable, just plug the cigarette lighter into the port or connect the battery terminals with alligator clips, and the connection is done in 3 minutes! Suitable for cars, trucks, boats, lawn mowers, motorcycles, trailers, dump trailers, tractors, RVs, vans, campers, ATVs, utility vehicle batteries, etc
Strong and durable material: Made of A+ monocrystalline solar cells with an efficiency of up to 21% 30%. Strong tempered glass + corrosion resistant ABS frame, can withstand extreme weather conditions such as UV rays, hail, sandstorms, up to 2400Pa wind pressure and 5400Pa snow load
Product Details| Brand: | Saillong |
|---|
| Model #: | SL-Saillong-0832 |
|---|
| Color: | Black |
|---|
| Dimensions: | 13.78 X 7.87 X 1.38 inches (Length X Width X Height) |
|---|
| Price: | $18.99 (Save 10%) |
|---|
Saillong 10W 12V Solar Car Battery Charger Maintainer, Waterproof Solar Panel Portable Solar Trickle Charger with Cigarette Lighter Plug Alligator Clip for Car Motorcycle Boat Marine RV
ECO-WORTHY Solar Battery Charger 12 Volt Waterproof Portable Power Solar Panel Solar Trickle Car Battery Charger Maintainer for Car Truck Boat RV Motorcycle Marine Trailer Battery,Plug and Play
Product Description[More Efficient] 30% high conversion rate crystalline silicon material + 95% high transmittance ETFE material, greatly improving the charging efficiency of solar chargers, which can achieve the most ideal charging effect in a limited space.
[Built-in Blocking Diode] The charging algorithm is upgraded, and the charging efficiency is increased by 20%-30%. Provides multiple protections such as overcharge protection, over discharge protection, reverse polarity protection, etc.
[Easier to Monitor] The solar panel junction box has indicators, which help visually monitor the working status.
[More Robust and Durable] This solar battery maintainer is covered with ultra clear PV glass which is more efficient, and also with Durable ABS plastic housing which makes this solar panels charger more stronger.
[Lightweight and Portable] The thickness is only 1/3 of the rigid panel; the weight is light and it is easy to carry. Comes with a SAE cable kit, plug and play, widely used in 12V batteries and DC charging equipment, such as cars, RVs, boats, etc.
Product Details| Brand: | ECO-WORTHY |
|---|
| Model #: | US-L02EP3BB18V-1 |
|---|
| Size: | 2.5W |
|---|
| Color: | Black |
|---|
| Dimensions: | 8.27 X 5.12 X 1.57 Inches (Length X Width X Height) |
|---|
| Price: | $17.99 |
|---|
ECO-WORTHY Solar Battery Charger 12 Volt Waterproof Portable Power Solar Panel Solar Trickle Car Battery Charger Maintainer for Car Truck Boat RV Motorcycle Marine Trailer Battery,Plug and Play
SUNER POWER Waterproof 30W 12V Solar Battery Charger & Maintainer PRO, Built-in UltraSmart MPPT Charge Controller, 12 Volt Solar Panel Trickle Charging Kits for Car Automotive Boat Marine RV Trailer
Product Description【Ultra-Smart MPPT Technology】This 12v solar panel battery charger is crafted with SUNER POWER's unique Ultra-Smart MPPT technology, the innovative MPPT ( Max Power Point Tracking) allows to deliver high tracking efficiency of up to 99% and peak conversion efficiency of 98%, improving approximately 20%-30% utilization rate than other competitors.
【Improved 3-stages Charging】The improved 3-Stages charging algorithm (Bulk, Absorption, Float) allows to charge 12-volt battery more effectively, ensures battery is safely recharged and increases battery life and performance.
【Free Maintenance】The solar trickle charger stops charging when battery has full charge, and automatically resumes when battery has discharge, totally free maintenance. A fully-automatic, worry-free and smart 12volt solar charger .
【 Visual Charging Level】The charge level indicators allow to visually monitor the stage of charge among 25%-50%-75%-100%.
【 Three Battery Charging Modes 】Independently charge and control each 12 volt battery with selectable charging modes - including 12V, 12V AGM and 12V Lithium - for all types of lead-acid boat, car, dump trailer, automotive, marine, RV, powersport, camper and deep cycle batteries, including flooded, gel, AGM, SLA, VRLA and maintenance-free, plus lifepo4 lithium batteries.
【Full Safety Protections】Built-in multiple protections including over-charge, over-discharge, over-voltage, over-current, over-load, short circuit, reverse polarity, and over-temperature, waterproof and spark-proof, the battery is fully protected.
Product Details| Brand: | SUNER POWER |
|---|
| Model #: | BC-30W-PB Ultra |
|---|
| Size: | BC-30W-PB Ultra |
|---|
| Dimensions: | 18.9 X 15.3 X 0.15 inches (Length X Width X Height) |
|---|
| Price: | $79.95 |
|---|
SUNER POWER Waterproof 30W 12V Solar Battery Charger & Maintainer PRO, Built-in UltraSmart MPPT Charge Controller, 12 Volt Solar Panel Trickle Charging Kits for Car Automotive Boat Marine RV Trailer
5W-Solar-Battery-Trickle-Charger-Maintainer 12V-Solar-Panel -Charger Waterproof Built-in Blocking Diode Small Solar Panels for Car, Automotive, Motorcycle, Boat, Marine, RV,Trailer, Truck, etc.

$18.90
@ Amazon.com (Save 17%)
Product Description📢High Conversion Rate▶ This 5W solar panel with monocrystalline A+ solar cell has an excellent cell efficiency of 21%-30%. Designed to charge and maintain 12V rechargeable batteries like LiFePO₄, Lithium Ion, AGM, SLA, GEL, EFB, MF, etc. Keep batteries in charged for trailer, tractor, truck, boat, motorcycle, RV, car, lawn mower, water pump, gate opener, electric fence, etc.
📢 5W Solar Panles ▶ 5W-Solar-Battery-Trickle-Charger-Maintainer 12V-Solar-Panel -Charger Waterproof Built-in Blocking Diode Solar Panel Trickle Charging Small Solar Panels for Car, Automotive, Motorcycle, Boat, Marine, RV,Trailer, Truck, etc
📢Built to Last▶ Low-iron tempered glass surface and corrosion-resistant aluminum frame, make this solar panel 100% waterproof and rustproof, and provide the prolonged lifespan up to 25 years. This 12V solar panel can withstand all weather conditions such as sandstorm, strong wind, thunderstorm, blizzard, hail, etc. It can withstand up to 2400Pa wind pressure and 5400Pa snow load.
📢Maintenance-Free▶ Solar charger can be left in place 24/7 to maintain 12V batteries, making it ideal for outdoor work, travel, or camping. It's easy to install and requires no maintenance. perfect for 12V batteries in cars, motorcycles, boats, snowmobiles, tractors, and more.
📢High Efficiency ▶ Over 23%monocrystalline cells A+ 5W MonocrystallinePortable Solar panel .Small size 12.6*6.3*0.78inches (320*160*20mm) ,5 years material and workmanship and 25 years power output ; , Free technical supportand customer service in USA.
📢Solar panel solar panels monocrystalline solar panel cheapest solar panels 5W Solar Panel 5 watt solar panel solar charger solar battery charger solar panel charger solar battery maintainer solar fence charger small solar panels boat battery charger
Product Details| Brand: | ACOPOWER |
|---|
| Model #: | HY-005-Mono |
|---|
| Size: | 5W |
|---|
| Color: | Mono |
|---|
| Dimensions: | 12.3 X 6.3 X 0.98 inches (Length X Width X Height) |
|---|
| Price: | $18.90 (Save 17%) |
|---|
5W-Solar-Battery-Trickle-Charger-Maintainer 12V-Solar-Panel -Charger Waterproof Built-in Blocking Diode Small Solar Panels for Car, Automotive, Motorcycle, Boat, Marine, RV,Trailer, Truck, etc.
SOLPERK 100W Solar Battery Charger Solar Battery Maintainer + Upgrade 10A MPPT Charge Controller + Adjustable Mount Bracket for Car RV Boat Motorcycle,12V Waterproof Solar Panel Kit Trickle Charging
Product Description【High-Efficiency MPPT Controller】This advanced MPPT charge controller providing over 30% additional power compared to standard solar charge controllers. This fully waterproof MPPT controller can be completely submerged in water. It offers comprehensive protection for your battery, safeguarding against overcharging, deep discharging, excessive voltage, current surges, overloads, short circuits, reverse polarity, and overheating
【Various of Application】 SOLPERK Solar Panel own monocrystalline A+ solar cell. Its efficiency is an industry-best 30%, generating 400 watt-hours of power per day in four hours of full sunlight.Suitable for RV, sheds, caravans, motorhomes, boats, trailers, etc
【Easy to install】Pre-drilled holes on the back and plug and play cable, solar panel and controller are connected, blue light is on, red light is on when battery is connected, green light is on when battery is fully charged
【Parcel Content】 Solar Panel kit includes 1PC 100W monocrystalline solar panel, 1PC 10A solar charge controller, 1PC19.7''Alligator Clip and 1 set of Z mounting brackets
【What You Get】 1 solar panel + 1 charger controller + 1 mounting bracket +1 alligator clips +1 O-rings + 1 set of mounting pieces. We provide one year after-sale service and lifetime technical support. 7×24 hours After-sales service ensures that your question is answered within one day.
Product Details| Brand: | SOLPERK |
|---|
| Model #: | 100W Solar Panel |
|---|
| Size: | 100W |
|---|
| Dimensions: | 12 X 8 X 1 inches (Length X Width X Height) |
|---|
| Price: | $149.99 |
|---|
SOLPERK 100W Solar Battery Charger Solar Battery Maintainer + Upgrade 10A MPPT Charge Controller + Adjustable Mount Bracket for Car RV Boat Motorcycle,12V Waterproof Solar Panel Kit Trickle Charging
OYMSAE 12W Solar Car Battery Charger Portable 12V Trickle Battery Charger & Maintainer Waterproof Solar Panel for Car Boat Automotive RV
Product DescriptionHigher Efficiency:PET has regular bumps on the surface. It forms secondary light transmission, absorb a lot of solar energy, and the photoelectric conversion efficiency is as high as 95%
Charging Visualization:The indicator lights of the solar charger help you better distinguish the usage status of the solar charger
Intelligent Protection:The solar panel has a built-in microprocessor. It provides multiple protections. It prevents reverse charging, and it is safely used for charging and maintaining 12V batteries
The package includes a three-piece SAE cable kit, plug, and play. Combiners and suction cups meet different installation needs. Suitable for cars, RVs, motorcycles, ships, snowmobiles, etc
More Durable:PET is waterproof and dust-proof. The PCB is used as the bottom plate, which is stronger and not easily broken (a broken panel will reduce the charging efficiency). If you have any questions, Feel free to contact us at any time
Product Details| Brand: | OYMSAE |
|---|
| Model #: | PO-M12W |
|---|
| Warranty: | 1 year |
|---|
| Size: | 12W |
|---|
| Color: | Blue |
|---|
| Dimensions: | 0.8 X 7.87 X 14.5 inches (Length X Width X Height) |
|---|
| Price: | $19.99 |
|---|
OYMSAE 12W Solar Car Battery Charger Portable 12V Trickle Battery Charger & Maintainer Waterproof Solar Panel for Car Boat Automotive RV
Renogy 200 Watt 12 Volt Portable Solar Panel with Waterproof 20A Charger Controller, Foldable 100W Solar Panel Suitcase with Adjustable Kickstand, Solar Charger for Power Station RV Camping Off Grid
Product DescriptionPlug and Play Solar Kit: Complete kit includes a 200W solar suitcase, a 20A waterproof Voyager charge controller, and alligator clips. Compatible with multiple kinds of 12V batteries, easily add to your existing system.
Remarkable Efficiency: Renogy solar panels using grade A+ monocrystalline solar cells. Advanced smart PWM technology charge controllers ensure charging efficiency and safety.
Foldable Design: Provide more flexible for outdoor and off-grid use, easy to carry, store and set up.
Safety Guarantee: Advanced smart PWM technology charge controller with multiple protections, ensure charging efficiency and safety.
Reailable Quality: This kit comes with a premium rugged canvas protective case. Highly weather-resistant backplanes are to last for 25 years. Additionally, we provide a 5-year materials and workmanship warranty.
Product Details| Brand: | Renogy |
|---|
| Model #: | 200 Watt 12 Volt Portable Solar Panel |
|---|
| Warranty: | Panel: 3-year material and workmanship warranty |
|---|
| Size: | 200W Panel-20A Controller |
|---|
| Color: | Monocrystalline |
|---|
| Dimensions: | 35.6 X 25.9 X 3.1 inches (Length X Width X Height) |
|---|
| Price: | $254.99 (Save 46%) |
|---|
Renogy 200 Watt 12 Volt Portable Solar Panel with Waterproof 20A Charger Controller, Foldable 100W Solar Panel Suitcase with Adjustable Kickstand, Solar Charger for Power Station RV Camping Off Grid